Medical PCB Printed Circuit Assembly Quotes
In the ever-evolving world of healthcare, medical devices continue to shrink in size while increasing in complexity and capability. At the heart of these devices lies a critical component — the printed circuit board manufacturing. As patient care depends more than ever on real-time monitoring and accurate diagnostics, the demand for high-quality medical printed circuit assembly has never been greater.
1. The Role of PCB Assembly in Medical Devices
From portable ECG monitors to implantable devices and diagnostic imaging systems, circuit board manufacturing form the foundation of nearly every electronic medical tool. These PCB board production must meet stringent industry standards such as ISO 13485 and IPC-A-610 Class III — ensuring they can perform flawlessly under critical conditions.

2. Key Considerations in Medical Electronics Assembly
( 1 )Miniaturization & Component Density
Medical PCB board fabrication often uses high-density interconnect (HDI) PCB designs to fit into small form-factor devices like hearing aids or insulin pumps.
( 2 ) PCB Material Selection
Circuit boards are often built using high-reliability substrates like FR4 High-Tg, polyimide, or ceramic for improved thermal performance and biocompatibility.
( 3 ) Precision Electronic Assembly Techniques
Techniques such as SMT with 01005 package placement, BGA reflow, and conformal coating are crucial to ensure durability and long-term performance.
( 4 ) Testing & Validation
Medical printed wiring board undergoes functional tests, AOI, X-ray inspection, and burn-in tests in specialized aging/test rooms to ensure zero-defect delivery.
3. Why Choose a Certified Medical PCB Assembly Manufacturer
When it comes to medical electronics, there is no room for error. Patient safety, diagnostic accuracy, and device reliability all hinge on the flawless performance of printed circuit board assemblies. Specialized printed circuit board manufacturers are equipped to meet the unique challenges of the healthcare industry, combining technical expertise with rigorous quality assurance systems.
( 1 ) Regulatory Compliance (FDA, ISO 13485)
Certified manufacturers adhere to international standards such as ISO 13485, which governs quality management systems for medical devices, and often comply with FDA requirements for Class I, II, or III medical equipment. These certifications demonstrate that the PCB fabrication manufacturer follows strict protocols for traceability, process control, and documentation, all of which are essential in regulated environments.
(2 ) Full Traceability of Components and Processes
Partnering with a qualified medical PCB assembler ensures complete traceability of materials and components throughout the circuit board manufacturing process. From the sourcing of RoHS-compliant components to the final functional testing phase, every action is logged and traceable. This not only enhances accountability but also supports faster diagnostics and corrective actions if any issues arise during a product’s lifecycle.
( 3 ) DFM and Reliability Engineering Support
Experienced PCB board manufacturers provide Design for Manufacturability (DFM) and reliability engineering support. These services help optimize the layout, circuit design, component selection, and PCB board assembly strategy for both performance and cost-efficiency. Reliability engineering goes even further by ensuring the final box build assembly can withstand harsh medical environments such as sterilization processes, body implantation, or long-term battery operation.
( 4 ) Scalable Production from Prototyping to Mass Production
Medical devices often undergo multiple design iterations before full commercialization. A certified circuit board manufacturer can support this entire journey — from engineering prototypes for clinical trials to high-volume runs for global deployment — while maintaining consistent quality and performance.
( 5 ) Customized Manufacturing Execution Systems (MES)
One of the greatest challenges in medical printed circuit board production is ensuring consistency across multiple production batches. Even minor deviations can result in significant downstream failures. To overcome this, PCB board makers often invest in customized assembly lines, automated inspection systems, and Manufacturing Execution Systems (MES) that provide real-time tracking of work-in-progress, material flow, and quality checkpoints.

4. Getting Accurate Medical PCB Assembly Quotes
Before launching your project, obtaining detailed and accurate medical PCB assembly quotes is crucial. A good quote should include:
- Breakdown of PCB fabrication, component sourcing, and assembly costs
- Lead time for prototypes and mass production
- Certification requirements (RoHS, ISO, etc.)
- Packaging and sterilization needs (if applicable)
Tips for a better quote experience:
- Provide Gerber files, BOM, and assembly drawings
- Specify testing requirements (e.g., ICT, functional testing)
SCSPCBA printed circuit assembly quotes show a PCB quote interface: upload Gerber files, select materials, input volume, and get a real-time price.
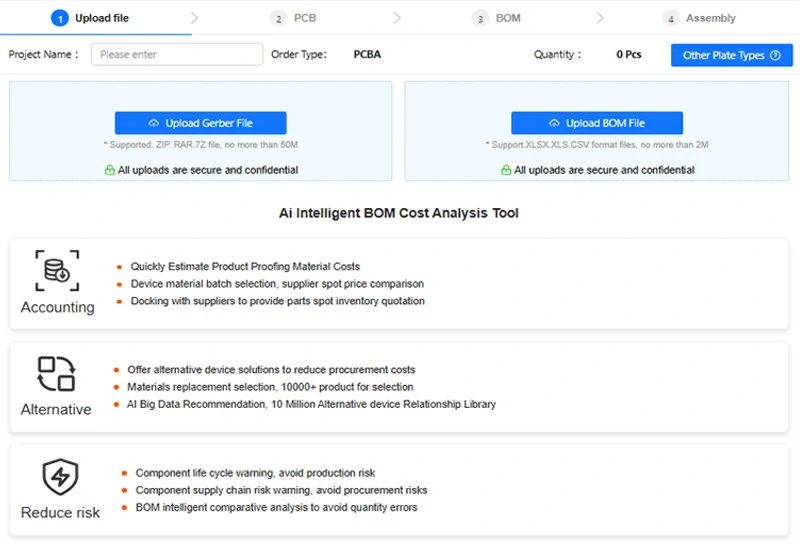
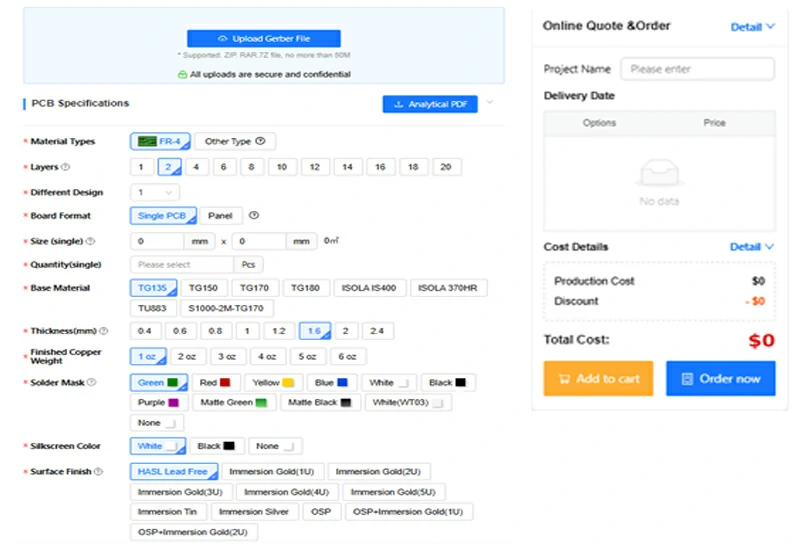
If you’re looking for a trusted partner for your next medical electronics project, contact our team today for a tailored quote and engineering consultation.











